
ในอุตสาหกรรมสุขภาพสมัยใหม่ อาหารเสริมในรูปแบบแคปซูลได้กลายเป็นส่วนสำคัญในชีวิตประจำวันของผู้คน ไม่ว่าจะเป็นวิตามิน แร่ธาตุ หรือสารสกัดจากสมุนไพร แคปซูลได้รับความนิยมเนื่องจากความสะดวก ความแม่นยำในการรับประทาน และความง่ายในการเก็บรักษา แล้วการผลิตอาหารเสริมในรูปแบบแคปซูลเป็นอย่างไร? บทความนี้จะวิเคราะห์กระบวนการผลิตอย่างครอบคลุม แคปซูลอาหารเสริม, ตั้งแต่การเลือกวัตถุดิบ การผลิตเทคโนโลยี ไปจนถึงการควบคุมคุณภาพ และข้อกำหนดทางกฎหมาย.
การเลือกวัตถุดิบและการออกแบบสูตร
การคัดเลือกและมาตรฐานของสารออกฤทธิ์
ส่วนผสมที่ออกฤทธิ์ ได้แก่ วิตามิน แร่ธาตุ (เช่น Mg2+, Zn2+, และ สารสกัดจากสมุนไพร (เช่น, เคอร์คูมิน, จินเซโนไซด์).
เกณฑ์การคัดเลือก:
- ความบริสุทธิ์สูง: ความบริสุทธิ์ของสารออกฤทธิ์หลักต้องมากกว่า 99% ($> 99\%$) เพื่อให้มั่นใจในความแม่นยำของการให้ยาและประสิทธิภาพที่คงที่.
- การรับรองแหล่งที่มา: ต้องให้ความสำคัญกับวัตถุดิบที่สามารถตรวจสอบย้อนกลับได้และได้รับการรับรอง เช่น วัตถุดิบที่ได้รับการรับรองมาตรฐานเกษตรอินทรีย์หรือการรับรองว่าปราศจากจีเอ็มโอ การรับรองเหล่านี้ช่วยรับประกันความปลอดภัย ความยั่งยืน และการปฏิบัติตามข้อกำหนดด้านสิ่งแวดล้อม.
- สารสกัดมาตรฐาน: สำหรับวัตถุดิบสมุนไพร ควรเลือกใช้สารสกัดมาตรฐานเพื่อให้มั่นใจว่ามีปริมาณสารออกฤทธิ์หลักที่คงที่ในทุกๆ ล็อต.
การเลือกสารช่วย (วัสดุเสริม)
สารช่วยเจือจางใช้เพื่อปรับรูปทรง, ทำให้การผลิตราบรื่น, และเพิ่มการดูดซึมทางชีวภาพ.
| ประเภทของวัสดุ | ตัวอย่างที่พบบ่อย | หน้าที่หลัก | การเปรียบเทียบ |
| ฟิลเลอร์ | ไมโครคริสตัลไลน์เซลลูโลส (MCC), แลคโตส | เพิ่มปริมาณให้กับเม็ดยา/แคปซูลเพื่อการบรรจุที่แม่นยำ. | MCC เป็นธรรมชาติ, ไม่ทำปฏิกิริยา, และมีคุณสมบัติการไหลที่ดีเยี่ยม. |
| สารหล่อลื่น | แมกนีเซียม สเตียเรต | ลดการเสียดสีระหว่างผงกับเครื่องจักร, ปรับปรุงประสิทธิภาพการผลิต. | ใช้กันอย่างแพร่หลาย แต่การใช้ในปริมาณสูงอาจส่งผลต่อเวลาการสลายตัว. |
| แคปซูลเปลือก | เจลาติน | แหล่งที่มาจากสัตว์, ต้นทุนต่ำ, มีความเสถียรดี. | ไม่เหมาะสำหรับผู้ทานมังสวิรัติ; ข้อพิจารณาด้านจริยธรรม. |
| เปลือกแคปซูลมังสวิรัติ HPMC | แหล่งที่มาจากพืช เหมาะสำหรับผู้ทานมังสวิรัติและข้อจำกัดทางศาสนา. | มีค่าใช้จ่ายสูงกว่าเล็กน้อย; มีความไวต่อความชื้นมากขึ้น. |
การพัฒนาสูตรและการทดสอบ
การคำนวณขนาดยา: กำหนดปริมาณของสารออกฤทธิ์อย่างแม่นยำตามการศึกษาทางคลินิกและปริมาณที่แนะนำต่อวัน (RDA) หรือปริมาณสูงสุดที่ร่างกายสามารถทนได้ (UL).
การประเมินความเข้ากันได้: ประเมินวัตถุดิบทั้งหมด (สารออกฤทธิ์และสารช่วย) ในสูตรเพื่อหาปฏิกิริยาเคมีหรือปฏิสัมพันธ์ทางกายภาพที่อาจเกิดขึ้น เพื่อป้องกันการเสื่อมสภาพของส่วนประกอบออกฤทธิ์.
การทดสอบความเสถียร
- การทดสอบการเสื่อมสภาพอย่างรวดเร็ว: ผลิตภัณฑ์ที่เสร็จสมบูรณ์จะถูกทดสอบภายใต้สภาวะสุดขั้ว (เช่น อุณหภูมิสูง 40℃ และความชื้นสูง 75% RH) เพื่อทำนายอายุการเก็บรักษาในระยะเวลาสั้น ๆ ซึ่งช่วยให้ประสิทธิภาพยังคงอยู่ที่อย่างน้อย 90% ของที่ระบุบนฉลากตลอดระยะเวลาการเก็บรักษา.
แนวโน้มความยั่งยืนและฉลากสะอาด
สูตรสมัยใหม่เน้นความยั่งยืนและ “ฉลากสะอาด”:
- การใช้ส่วนผสมจากพืช: เพิ่มการใช้สารสกัดจากพืชและโปรตีนจากพืชให้มากที่สุด โดยแทนที่ส่วนประกอบที่ได้จากสัตว์.
- การลดการพึ่งพาคอลลาเจนจากสัตว์: เปลี่ยนไปใช้ส่วนผสมที่ส่งเสริมการผลิตคอลลาเจนภายในร่างกาย (เช่น วิตามินซี, ไบโอติน) หรือทางเลือกที่เป็นวีแกนเพื่อตอบสนองความต้องการของผู้บริโภคที่รับประทานมังสวิรัติและคำนึงถึงจริยธรรม.
กระบวนการผลิตอาหารเสริมแคปซูล
กระบวนการผลิตแคปซูลเกี่ยวข้องกับการบรรจุเปลือกที่เตรียมไว้ล่วงหน้าด้วยผงหรือเม็ด โดยยึดตามมาตรฐาน GMP (Good Manufacturing Practice) อย่างเคร่งครัด.
🏅 การเตรียมวัตถุดิบและการบำบัดเบื้องต้น
- การชั่งน้ำหนัก: ส่วนผสมที่ออกฤทธิ์ทั้งหมดและสารช่วยเจือจางถูกชั่งน้ำหนักอย่างแม่นยำตามสูตรการผลิตในแต่ละล็อต.
- การบดและการร่อน วัสดุถูกบดให้มีความละเอียดตามที่ต้องการและผ่านตะแกรงเพื่อกำจัดก้อนและสิ่งสกปรกออก ทำให้มั่นใจได้ถึงการกระจายขนาดอนุภาคที่สม่ำเสมอ.
🧪 ผสมผสาน
- การผสมแบบแห้ง: วัสดุผงทั้งหมดที่ผ่านการชั่งน้ำหนักและแปรรูปแล้วจะถูกผสมให้เข้ากันอย่างสม่ำเสมอในเครื่องผสมเฉพาะทาง (เช่น เครื่องผสมรูปตัววี, เครื่องผสมสามมิติ) การบรรลุความสม่ำเสมอเป็นสิ่งสำคัญอย่างยิ่งเพื่อให้มั่นใจในความแม่นยำและความสม่ำเสมอของปริมาณในแต่ละแคปซูล.
- การเติมสารหล่อลื่น: สารหล่อลื่น (เช่น แมกนีเซียมสเตียเรต) ถูกเติมในขั้นตอนสุดท้ายของการผสมเพื่อเพิ่มความสามารถในการไหลของผง ซึ่งเป็นสิ่งสำคัญสำหรับการบรรจุที่มีประสิทธิภาพ.
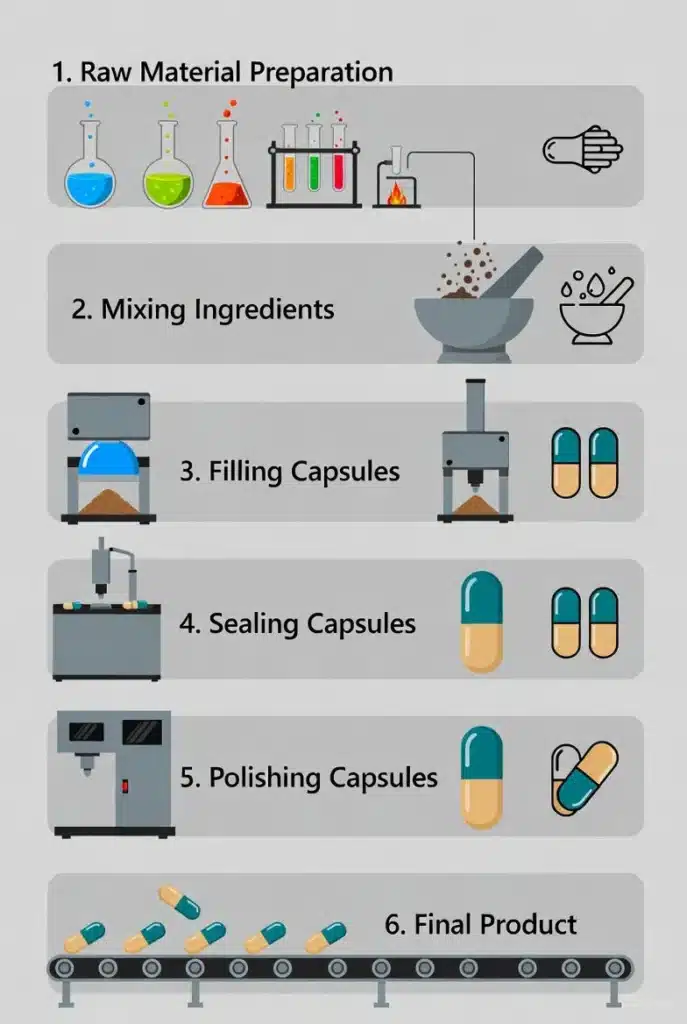
💊 การห่อหุ้ม
- การแยกเปลือกแคปซูล เครื่องบรรจุแคปซูลอัตโนมัติหรือกึ่งอัตโนมัติจะเปิดและแยกเปลือกแคปซูลที่ขึ้นรูปไว้ล่วงหน้าออกจากกัน.
- การบรรจุผง: ผงที่ผสมอย่างสม่ำเสมอจะถูกบรรจุลงในตัวล่าง (หรือภาชนะ) ของเปลือกแคปซูล.
- ปิดและล็อก: ส่วนบน (ฝา) ของแคปซูลถูกเชื่อมต่อกลับเข้ากับส่วนล่างของตัวแคปซูลและล็อคแน่นหนา เพื่อให้แน่ใจว่าสามารถกักเก็บเนื้อหาภายในได้และป้องกันการรั่วไหล.
✨ การขัดเงาและการตรวจสอบ
- การขัดเงา: เครื่องขัดแคปซูลใช้สำหรับเช็ดผงตกค้างออกจากผิวแคปซูล เพื่อให้ได้ผิวที่เรียบเนียนและสะอาด.
- การตรวจสอบน้ำหนัก ตัวอย่างแบบสุ่มจะถูกตรวจสอบเพื่อหาความแปรปรวนของน้ำหนักแคปซูล เพื่อยืนยันความสม่ำเสมอและความถูกต้องของปริมาณการบรรจุ.
- การตรวจจับโลหะ: ผลิตภัณฑ์สุดท้ายจะถูกคัดกรองเพื่อหาสิ่งปนเปื้อนโลหะที่อาจเกิดขึ้นได้ เพื่อให้มั่นใจในความปลอดภัยของผู้บริโภค.
📦 การประมวลผลหลังการถ่ายทำ
- บรรจุภัณฑ์หลัก: แคปซูลที่เสร็จสมบูรณ์จะถูกบรรจุลงในภาชนะที่บรรจุผลิตภัณฑ์โดยตรง (เช่น ขวด, แผงบรรจุ).
- บรรจุภัณฑ์รอง: นี่คือการนำบรรจุภัณฑ์หลักไปใส่ในกล่องกระดาษ, ติดฉลากครั้งสุดท้าย, และบรรจุกล่องเพื่อการขนส่ง.
- การทดสอบและการปล่อยผลิตภัณฑ์สำเร็จรูป: การตรวจสอบคุณภาพครั้งสุดท้าย รวมถึงการทดสอบทางฟิสิกส์เคมีและจุลชีววิทยา จะดำเนินการ การปล่อยสินค้าล็อตนั้นเพื่อการจัดจำหน่ายจะเกิดขึ้นได้ก็ต่อเมื่อได้รับการยืนยันว่ามีการปฏิบัติตามข้อกำหนดคุณภาพทั้งหมดอย่างครบถ้วนแล้วเท่านั้น.
ข้อกำหนดด้านอุปกรณ์และสิ่งอำนวยความสะดวก
การผลิตอาหารเสริมคุณภาพสูงต้องอาศัยอุปกรณ์ที่ทันสมัยและการจัดวางสถานที่ผลิตที่เป็นไปตามข้อกำหนดของหลักเกณฑ์วิธีการที่ดีในการผลิต (GMP).
I. รายการอุปกรณ์การผลิตหลัก
ตารางต่อไปนี้แสดงรายการอุปกรณ์ที่จำเป็นสำหรับสายการผลิตแคปซูลหรือเม็ดยา โดยเน้นที่ฟังก์ชันและช่วงราคา.
| ชื่ออุปกรณ์ | ฟังก์ชันหลัก | ช่วงราคา (หยวน) |
| เครื่องปั่น (เช่น แบบตัววี, เครื่องผสมสามมิติ) | ตรวจสอบให้แน่ใจว่ามีการผสมผงวัตถุดิบอย่างสม่ำเสมอ | ¥ 20,000 – 150,000 |
| เครื่องบรรจุแคปซูลหรือเครื่องอัดเม็ดยาความเร็วสูง | การขึ้นรูปขนาดยา (บรรจุผงหรืออัดเม็ด) | ¥ 100,000 – 500,000 |
| อุปกรณ์อบแห้ง (เช่น เครื่องอบแห้งแบบเตียงไหล) | การอบแห้งเม็ดเปียกอย่างรวดเร็วและสม่ำเสมอ (สำหรับการทำเม็ดเปียก) | ¥ 80,000 – 300,000 |
| เครื่องบรรจุภัณฑ์อัตโนมัติ (บรรจุขวด/บรรจุแผง) | บรรจุภัณฑ์ผลิตภัณฑ์ขั้นสุดท้าย, ตรวจสอบความสมบูรณ์ของการปิดผนึก | ¥ 100,000 – 400,000 |
| เครื่องมือตรวจจับคุณภาพ (เช่น เครื่องสแกนเอกซเรย์) | ตรวจสอบน้ำหนักและความแข็งของผลิตภัณฑ์ และตรวจจับโลหะหรือสิ่งปนเปื้อน | ¥ 150,000 – 800,000 |
II. การจัดวางสิ่งอำนวยความสะดวกและการควบคุมสิ่งแวดล้อม
สถานที่ต้องถูกแบ่งแยกตามหลักวิทยาศาสตร์ตามกระบวนการทำงาน และควบคุมตัวแปรทางสิ่งแวดล้อมอย่างเคร่งครัดเพื่อป้องกันการปนเปื้อนข้าม.
- การจัดโซนห้องสะอาด
- พื้นที่วัตถุดิบ: พื้นที่เฉพาะสำหรับการรับ ตรวจสอบ และเก็บรักษาวัสดุ.
- พื้นที่การผลิต (โซนสะอาด): พื้นที่การผลิตหลักที่ต้องรักษาความสะอาดในระดับสูง (เช่น ระดับ D หรือ C).
- พื้นที่บรรจุภัณฑ์: พื้นที่ที่กำหนดไว้สำหรับการปิดผนึกขั้นสุดท้าย การบรรจุหีบห่อ และการติดฉลากสินค้าสำเร็จรูป.
- ระบบกรองอากาศ: จำเป็นต้องติดตั้งระบบกรองอากาศ HEPA (High-Efficiency Particulate Air Filter) ที่มีประสิทธิภาพเพื่อรับรองความสะอาดของอากาศในพื้นที่การผลิต โดยควบคุมการปนเปื้อนของอนุภาคและจุลินทรีย์.
III. การเลือกขนาดและการประมาณการลงทุน
- ตัวเลือกขนาด:
- เกรดห้องปฏิบัติการ: ใช้เครื่องมือแบบแมนนวลหรือกึ่งอัตโนมัติ เหมาะสำหรับการทดสอบสูตรขนาดเล็กและการวิจัยและพัฒนา.
- เกรดอุตสาหกรรม: ใช้สายการผลิตอัตโนมัติเต็มรูปแบบ โดยมีการลงทุนโดยทั่วไปเกิน 500,000 ถึง 2 ล้านหยวน มุ่งเน้นที่ความจุสูงและต้นทุนต่อหน่วยต่ำ.
- ประมาณการค่าใช้จ่าย:
- เงินทุนเริ่มต้นสำหรับสายการผลิตขนาดเล็ก (รวมถึงอุปกรณ์พื้นฐานและการจัดตั้งห้องสะอาดอย่างง่าย) อยู่ที่ประมาณ 100,000 ถึง 500,000 หยวน.
- ภายใต้การดำเนินงานที่สมเหตุสมผล ระยะเวลาคืนทุน (ROI) สำหรับสายการผลิตขนาดเล็กโดยทั่วไปจะอยู่ที่ 1 ถึง 2 ปี.
การควบคุมคุณภาพและการปฏิบัติตามข้อกำหนด
การควบคุมคุณภาพอย่างเข้มงวด (QC) และการปฏิบัติตามกฎระเบียบอย่างครบถ้วนเป็นรากฐานของความน่าเชื่อถือและความสามารถในการแข่งขันในตลาดของแบรนด์ผลิตภัณฑ์เสริมอาหารทุกประเภท.
I. การควบคุมคุณภาพแบบครบวงจร (QC)
การควบคุมคุณภาพต้องถูกผสานรวมไว้ในทุกขั้นตอนของการผลิตเพื่อให้แน่ใจว่าผลิตภัณฑ์มีความปลอดภัยและมีประสิทธิภาพ.
- การตรวจสอบระหว่างกระบวนการ (IPM):
- น้ำหนักและขนาดยา: การตรวจสอบแบบเรียลไทม์ของน้ำหนักเฉลี่ยและการเปลี่ยนแปลงของน้ำหนักของแคปซูลหรือเม็ดยาเพื่อรับประกันความถูกต้องของการให้ยา.
- เวลาการสลายตัว: การทดสอบตัวอย่างเพื่อหาเวลาที่เม็ดยาหรือแคปซูลใช้ในการสลายตัวในของเหลวที่กำหนด ซึ่งโดยทั่วไปจะต้องน้อยกว่า 30 นาที เพื่อให้แน่ใจว่าสารออกฤทธิ์ถูกปล่อยออกมา.
- การทดสอบการปล่อยชุด:
- ขีดจำกัดของจุลินทรีย์: ทดสอบ $E. coli$ เชื้อรา และยีสต์ เพื่อให้แน่ใจว่าผลิตภัณฑ์ปราศจากการปนเปื้อนของจุลินทรีย์.
- โลหะหนัก: การทดสอบสารตะกั่ว (Pb), สารหนู (As), สารแคดเมียม (Cd), และสารปรอท (Hg). ระดับต้องอยู่ต่ำกว่าขีดจำกัดความปลอดภัยของประเทศอย่างมาก.
- อัตราการละลาย: การรับรองว่าสารออกฤทธิ์จะละลายในอัตราและขอบเขตที่คาดหวังในระบบย่อยอาหารของมนุษย์เพื่อการดูดซึมที่เหมาะสม.

- การตรวจสอบระหว่างกระบวนการ (IPM):
- น้ำหนักและขนาดยา: การตรวจสอบแบบเรียลไทม์ของน้ำหนักเฉลี่ยและการเปลี่ยนแปลงของน้ำหนักของแคปซูลหรือเม็ดยาเพื่อรับประกันความถูกต้องของการให้ยา.
- เวลาการสลายตัว: การทดสอบตัวอย่างเพื่อหาเวลาที่เม็ดยาหรือแคปซูลใช้ในการสลายตัวในของเหลวที่กำหนด ซึ่งโดยทั่วไปจะต้องน้อยกว่า 30 นาที เพื่อให้แน่ใจว่าสารออกฤทธิ์ถูกปล่อยออกมา.
- การทดสอบการปล่อยชุด:
- ขีดจำกัดของจุลินทรีย์: ทดสอบ $E. coli$ เชื้อรา และยีสต์ เพื่อให้แน่ใจว่าผลิตภัณฑ์ปราศจากการปนเปื้อนของจุลินทรีย์.
- โลหะหนัก: การทดสอบสารตะกั่ว (Pb), สารหนู (As), สารแคดเมียม (Cd), และสารปรอท (Hg). ระดับต้องอยู่ต่ำกว่าขีดจำกัดความปลอดภัยของประเทศอย่างมาก.
- อัตราการละลาย: การรับรองว่าสารออกฤทธิ์จะละลายในอัตราและขอบเขตที่คาดหวังในระบบย่อยอาหารของมนุษย์เพื่อการดูดซึมที่เหมาะสม.
II. กรอบการกำกับดูแลและข้อกำหนดในการติดฉลาก
แบรนด์ต้องดำเนินการภายใต้กรอบการกำกับดูแลของตลาดโลกที่สำคัญ.
- ข้อบังคับของจีน: การปฏิบัติตามอย่างเคร่งครัดต่อมาตรฐานการผลิตอาหารเสริมสุขภาพตามข้อกำหนดของระบบการผลิตที่ดี (GMP) ของประเทศจีน ซึ่งกำหนดข้อกำหนดที่เฉพาะเจาะจงสำหรับการผลิต การทดสอบ และการบันทึกข้อมูล.
- มาตรฐานสากล: การปฏิบัติตามข้อกำหนดของตลาดระหว่างประเทศ เช่น มาตรฐาน cGMP ของสำนักงานคณะกรรมการอาหารและยาของสหรัฐอเมริกา (US FDA) และของสหภาพยุโรป REACH ข้อบังคับ (การจดทะเบียน การประเมิน การอนุญาต และการจำกัดการใช้สารเคมี).
- ข้อกำหนดในการติดฉลาก: ข้อมูลบนฉลากต้องเป็นความจริงและถูกต้อง รวมถึง:
- รายการส่วนผสมและปริมาณที่แม่นยำ.
- คำเตือนสารก่อภูมิแพ้ (เช่น กลูเตน ถั่วเหลือง ถั่วเปลือกแข็ง).
- คำกล่าวอ้างเกี่ยวกับสุขภาพและคำเตือนสำหรับกลุ่มประชากรที่ไม่เหมาะสม.
III. ความเสี่ยงทั่วไปและมาตรการป้องกัน
| ปัญหาความเสี่ยง | สาเหตุที่แท้จริง | วิธีแก้ไข/มาตรการป้องกัน |
| การปนเปื้อนของจุลินทรีย์ | การคงเหลือวัตถุดิบ, คุณภาพอากาศในห้องสะอาดไม่ดี | การฆ่าเชื้อด้วยแสง UV อย่างสม่ำเสมอในสภาพแวดล้อมการผลิต; มาตรการสุขอนามัยที่เข้มงวดสำหรับพนักงาน. |
| ความล้มเหลวของความเสถียร | สารออกฤทธิ์เสื่อมสภาพเนื่องจากความชื้น แสง หรือความร้อน | ใช้วัสดุบรรจุภัณฑ์ที่มีคุณสมบัติกั้นอากาศสูง (เช่น บรรจุภัณฑ์อลูมิเนียมแบบบลิสเตอร์) บรรจุภัณฑ์แบบสุญญากาศหรือบรรจุภัณฑ์ที่ไล่อากาศออกด้วยไนโตรเจน และจัดเก็บในอุณหภูมิและความชื้นที่ควบคุมได้. |
| การปนเปื้อนของสิ่งแปลกปลอม | การสึกหรอของอุปกรณ์, มลพิษจากภายนอก | ใช้เครื่องตรวจจับรังสีเอกซ์/โลหะสำหรับการสแกนแบบต่อเนื่อง; กำหนดตารางการบำรุงรักษาและสอบเทียบอุปกรณ์ตามกำหนด. |
กรณีศึกษาบทเรียน: เหตุการณ์ข้าวหมักยีสต์แดงของบริษัทโคบายาชิ ฟาร์มาซูติคอลในญี่ปุ่นชี้ให้เห็นว่า แม้แต่ส่วนผสมจากธรรมชาติ หากผลิตหรือเก็บรักษาอย่างไม่เหมาะสม ก็สามารถสร้างสารเมตาบอไลต์ที่เป็นอันตราย (เช่น ซิตรินินที่ปนเปื้อนโดยไม่ตั้งใจ) ซึ่งนำไปสู่ปัญหาสุขภาพที่แพร่หลายและการเรียกคืนผลิตภัณฑ์ บริษัทต่างๆ จำเป็นต้องเสริมสร้างการตรวจสอบทางพิษวิทยาของ วัตถุดิบ ห่วงโซ่อุปทานและผลิตภัณฑ์ระหว่างกลาง.
IV. เส้นทางสู่การรับรองและความไว้วางใจในแบรนด์
การรับรองเป็นวิธีที่มีประสิทธิภาพสูงในการได้รับการยอมรับจากตลาดและความไว้วางใจจากผู้บริโภค.
- ประกาศนียบัตรการจัดการพื้นฐาน การได้รับการรับรองมาตรฐาน ISO 22000 (ระบบการจัดการความปลอดภัยด้านอาหาร) เป็นการสร้างกรอบการจัดการความเสี่ยงอย่างเป็นระบบ.
- การรับรองความน่าเชื่อถือระดับพรีเมียม: การดำเนินการขอรับรองมาตรฐานออร์แกนิก, โคเชอร์ และฮาลาล เพื่อสร้างความแตกต่างให้กับผลิตภัณฑ์ เพิ่มความน่าเชื่อถือของแบรนด์อย่างมีนัยสำคัญ และเสริมสร้างความสามารถในการแข่งขันในระดับสากล.
ความท้าทาย นวัตกรรม และแนวโน้มในอนาคต
I. ความท้าทายหลัก
อุตสาหกรรมกำลังเผชิญกับอุปสรรคหลายประการในทันที:
- ความผันผวนของวัตถุดิบ การหยุดชะงักของห่วงโซ่อุปทาน และความไม่มั่นคงทางภูมิรัฐศาสตร์มักนำไปสู่ความไม่แน่นอนของราคาและความพร้อมใช้งานของวัตถุดิบหลัก.
- แรงกดดันจากสิ่งแวดล้อม: ความต้องการของผู้บริโภคที่เพิ่มขึ้นและกฎระเบียบสำหรับ ทางเลือกแทนพลาสติก กำหนดให้มีการเปลี่ยนไปสู่โซลูชันบรรจุภัณฑ์ที่ยั่งยืน.
- การผลิตแบบเฉพาะบุคคล ความจำเป็นในการขยายขีดความสามารถเพื่อ อาหารเสริมตามความต้องการและปรับแต่งเฉพาะบุคคล เพื่อตอบสนองความต้องการทางพันธุกรรมหรืออาหารของแต่ละบุคคล.
II. ปัจจัยขับเคลื่อนนวัตกรรม
เทคโนโลยีและวิทยาศาสตร์กำลังขับเคลื่อนนวัตกรรมสำคัญ:
- สูตรที่ได้รับการปรับให้เหมาะสมด้วยปัญญาประดิษฐ์ การใช้ปัญญาประดิษฐ์ (AI) เพื่อวิเคราะห์ข้อมูลอย่างรวดเร็วและปรับสัดส่วนส่วนผสมให้เหมาะสมที่สุดเพื่อเพิ่มประสิทธิภาพและความเสถียร.
- นาโนเทคโนโลยี: การใช้เทคนิคการห่อหุ้มระดับนาโนเพื่อปกป้องสารออกฤทธิ์ที่ไวต่อสภาพแวดล้อมและเพิ่มอัตราการดูดซึมและความสามารถในการออกฤทธิ์ทางชีวภาพอย่างมีนัยสำคัญ.
- วัสดุที่ยั่งยืน: พัฒนาสารช่วยในการผลิตและบรรจุภัณฑ์ที่เป็นมิตรต่อสิ่งแวดล้อมขั้นสูง เช่น เปลือกแคปซูลที่ทำจากสาหร่ายทะเลแทนเจลาตินหรือ HPMC แบบดั้งเดิม.
III. แนวโน้มในอนาคต
- ภูมิทัศน์ตลาดหลังปี 2025: ตลาดจะถูกครอบงำโดยโรงงานอัจฉริยะมากขึ้น ซึ่งมีลักษณะเด่นคือการอัตโนมัติเต็มรูปแบบและการตรวจสอบคุณภาพแบบเรียลไทม์.
- การครองตลาดของ OEM ในธุรกิจอีคอมเมิร์ซ รูปแบบการผลิตสินค้าตามคำสั่ง (OEM) สำหรับพาณิชย์อิเล็กทรอนิกส์จะกลายเป็นเส้นทางหลักสำหรับการเปิดตัวผลิตภัณฑ์อย่างรวดเร็วและการจัดหาสินค้าที่สามารถปรับขนาดได้ เพื่อตอบสนองความต้องการของผู้บริโภคทั่วโลกโดยตรง.
สรุป
โครงการนี้ได้กำหนดโครงสร้างพื้นฐานที่แข็งแกร่งซึ่งจำเป็นต่อความสำเร็จในภาคธุรกิจอาหารเสริมสุขภาพ เราได้กำหนดข้อกำหนดที่เข้มงวดสำหรับการจัดหาวัตถุดิบคุณภาพสูง การออกแบบโรงงานที่สอดคล้องกับมาตรฐาน GMP และการควบคุมคุณภาพอย่างครอบคลุม การผลิตแคปซูลที่สอดคล้องกับมาตรฐานและมีคุณภาพสูงไม่ใช่เพียงแค่ขั้นตอนการผลิต แต่เป็นรากฐานของความไว้วางใจและการเติบโตของอุตสาหกรรมสุขภาพ เราพร้อมแล้วที่จะก้าวจากการวางแผนไปสู่การปฏิบัติ.
เมื่อได้เข้าใจรายละเอียดของกระบวนการผลิตแล้ว ขั้นตอนต่อไปคือการนำกระบวนการนี้ไปไว้ในบริบททางธุรกิจและกฎระเบียบที่กว้างขึ้น เราขอแนะนำให้อ่าน สำหรับคำแนะนำที่ครอบคลุมตั้งแต่แนวคิดจนถึงการเข้าสู่ตลาด.



